Abstract
Selinexor is the first in class, orally available, exportin inhibitor, approved for heavily pretreated, triple class refractory myeloma patients (pts) in combination with dexamethasone (Sd) and in pts with relapsed/refractory myeloma (RRMM) with 1-3 prior lines of therapy in combination with bortezomib and dexamethasone (SVd). In clinical practice, the use of selinexor is associated with gastrointestinal toxicity, appetite loss and fatigue which require aggressive management, and may limit its use, especially outside the context of a clinical trial. In this report we sought to provide "real world” evidence for the efficacy and clinical use of selinexor combinations, as well as the post-progression therapies and their outcome.
The analysis included consecutive pts with RRMM treated with Sd or SVd in the Department of Clinical Therapeutics, Athens, Greece. This is a retrospective study based on prospective collection of efficacy and safety data per our institution's policy. The management of GI toxicity was based on the guidance provided by experts in the use of selinexor (Gavriatopoulou M et al, Leukemia 2020).
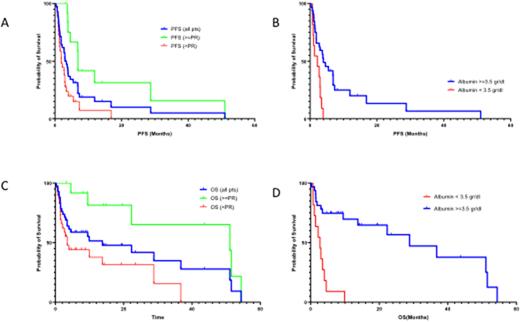
We analyzed 44 pts with a median of 6 prior lines (range 2-14); median time from diagnosis was 6 years (range 2-15). All pts were refractory to lenalidomide and exposed to PIs, 91% were refractory to ≥one PI (70% to carfilzomib), 86% to pomalidomide, 93% to anti-CD38 monoclonal antibody and 34% (15/44) to anti-BCMA therapy. Median age was 69 years (range 40-89), 25% had hemoglobin <10 g/dl, 18% platelet counts <100x109/L, LDH >ULN in 41%, 21% had creatinine >1.5 mg/dl, 27% had serum albumin <3.5 gr/dl, ISS-3 in 24% and 29% had high risk cytogenetics. Regimens were Sd in 21 (48%) and SVd in 23 (52%) pts. Starting dose was 40-100 mg per week in 16 (37%) and >100 mg week in 28 (63%) pts. Median duration of selinexor therapy was 2.7 months. On ITT, ORR was 29.5% (13/44 pts, CR: 2, VGPR: 3, PR:8): 35% for SVd and 24% for Sd. In anti-BCMA pretreated pts, ORR was 13% (2/15). Median time to first response was one month (range 0.8-2.9). Median PFS was 3.0 months for all pts and 6.9 months for responders (≥PR; Fig 1A). Median PFS was 2.7 months for Sd treated pts and 3.4 months for SVd treated pts; it was 4 vs 2.7 months for pts starting with ≤100 mg vs >100 mg per week (p=0.163).
A dose reduction for selinexor was required in 25/44 (56%) pts; in 37.5% of those starting at <100 mg/week and in 70% of those starting at ≥100 mg; dose reduction was not associated with worse PFS (median 3.8 months vs 1.9 for pts not requiring dose reduction).
In univariate analysis, factors associated with inferior PFS were serum albumin <3.5 g/dl (2.1 vs 4.6 months, p=0.001) and LDH >ULN (2.7 vs 3.97, p=0.032), but not the number of prior lines and prior exposure to anti-BCMA therapy (Fig 1B).
The median OS was 13.7 months (16 pts remain alive); it was 28 months for those with ≥PR and 4 months for non-responding pts (Fig 1C). In univariate analysis serum albumin <3.5 g/dl (2.1 vs 36.7 months, p<0.001) and LDH >ULN (3.4 vs 36.7, p=0.024) were associated with worse OS (1D). In a sensitivity analysis, there was no interaction of serum albumin levels with the starting dose of selinexor, dose reduction or prior lines of therapy.
After progression to Sd/SVd, 20 pts received further therapy; they had a median of 7 prior lines (range 5-13) and their treatment included belantamab mafodotin in 4, anti-CD38-containing in 2, PIs+/- chemo in 6, PIs with IMiDs in 2, IMiDs +/- chemo in 3, Sd with added PI in 2 and one received chemo alone. On ITT, the ORR was 40% (8/20) and the PFS was 3.4 months.
The most common toxicities were infections (25%, Gr3-5 in 16%), thrombocytopenia (55%, Gr3-4: 23%), fatigue (66%, Gr3-4: 14%), nausea (47%, Gr3-4: 9%), diarrhea (9%, Gr3-4 in 2.5%), hyponatriemia (11%, Gr3-4: 9%) and symptoms of confusion/altered mental state (18%, Gr3 in 7%).
In conclusion, outside the context of clinical trials, the efficacy of selinexor combinations in heavily pretreated RRMM pts is moderate, with responding pts having a higher probability for longer remissions. OS in this heavily pretreated population, however, exceeds 1 year and sets the benchmark for the evaluation of new therapies. Dose adjustments are required in most pts indicating that optimal dose of selinexor may be lower than the recommended. The prognostic impact of low serum albumin in these pts needs further evaluation as it may be a marker of poor nutrition, advanced disease and poor tolerance to selinexor.
Disclosures
Kastritis:Genesis: Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Takeda: Honoraria; Janssen: Consultancy, Honoraria, Research Funding; GSK: Honoraria. Gavriatopoulou:Janssen Cilag: Honoraria; Sanofi: Honoraria; Genesis Pharma: Honoraria; Amgen: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; GSK: Consultancy, Honoraria; Takeda: Consultancy, Honoraria. Terpos:Sanofi: Honoraria, Research Funding; Takeda: Honoraria, Other: Travel expenses, Research Funding; Novartis: Honoraria; Janssen: Honoraria, Research Funding; GSK: Honoraria, Research Funding; Genesis: Honoraria, Research Funding; EUSA Pharma: Honoraria, Other: Travel expenses; BMS: Honoraria; Amgen: Honoraria, Other: Travel expenses, Research Funding. Dimopoulos:BMS: Honoraria; Amgen: Honoraria; Takeda: Honoraria; Beigene: Honoraria; Janssen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal